<!–

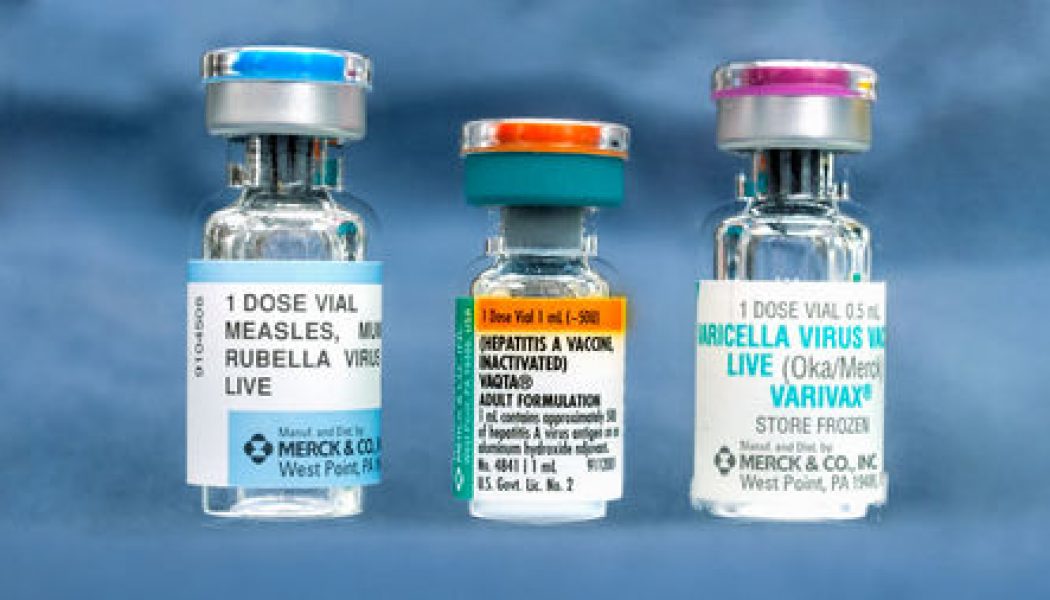
Several vaccines on the U.S. vaccination schedule are made in cells from fetuses aborted decades ago. They include vaccines against rubella, hepatitis A, and chicken pox.
J. Cohen/Science
Science’s COVID-19 reporting is supported by the Pulitzer Center.
Senior Catholic leaders in the United States and Canada, along with other antiabortion groups, are raising ethical objections to promising COVID-19 vaccine candidates that are manufactured using cells derived from human fetuses electively aborted decades ago. They have not sought to block government funding for the vaccines, which include two candidate vaccines that the Trump administration plans to support with an investment of up to $1.7 billion, as well as a third candidate made by a Chinese company in collaboration with Canada’s National Research Council (NRC). But they are urging funders and policymakers to ensure that companies develop other vaccines that do not rely on such human fetal cell lines and, in the United States, asking the government to “incentivize” firms to only make vaccines that don’t rely on fetal cells.
“It is critically important that Americans have access to a vaccine that is produced ethically: no American should be forced to choose between being vaccinated against this potentially deadly virus and violating his or her conscience,” members of the U.S. Conference of Catholic Bishops and 20 other religious, medical, and political organizations that oppose abortion wrote to Stephen Hahn, commissioner of the U.S. Food and Drug Administration (FDA), in April. “Thankfully, other [COVID-19] vaccines … utilize cell lines not connected to unethical procedures and methods.”
Related
“We urge your government to fund the development of vaccines that do not create an ethical dilemma for many Canadians,” wrote Archbishop of Winnipeg Richard Gagnon, president of the Canadian Conference of Catholic Bishops, and 17 other antiabortion religious, medical, and politic groups and individuals in a 21 May letter to Prime Minister Justin Trudeau. “The … manufacture of vaccines using such ethically-tainted human cell lines demonstrates profound disrespect for the dignity of the human person.”
FDA and senior White House officials did not respond to emails requesting comment on the letter to Hahn. In Canada, the health ministry has promised to respond to the letter to Trudeau, says Moira McQueen, executive director of the Canadian Catholic Bioethics Institute and lead signatory on the letter.
Cells derived from elective abortions have been used since the 1960s to manufacture vaccines, including current vaccines against rubella, chickenpox, hepatitis A, and shingles. They have also been used to make approved drugs against diseases including hemophilia, rheumatoid arthritis, and cystic fibrosis. Now, research groups around the world are working to develop more than 130 candidate vaccines against COVID-19, according to the World Health Organization; 10 had entered human trials as of 2 June.
At least five of the candidate COVID-19 vaccines use one of two human fetal cell lines: HEK-293, a kidney cell line widely used in research and industry that comes from a fetus aborted in about 1972; and PER.C6, a proprietary cell line owned by Janssen, a subsidiary of Johnson & Johnson, developed from retinal cells from an 18-week-old fetus aborted in 1985. Both cell lines were developed in the lab of molecular biologist Alex van der Eb at Leiden University. Two of the five vaccines have entered human trials (see table, below).
| Developer | Vaccine type | Fetal cells used | Human trials | Potential U.S. funding | Warp Speed pick |
|---|---|---|---|---|---|
| CanSino Biologics, Inc./Beijing Institute of Biotechnology | Replication-deficient adenovirus | HEK-293 | Yes (phase II) | No | No |
| University of Oxford/AstraZeneca | Replication-deficient adenovirus | HEK-293 | Yes (phase II/III) | $1.2 billion | Yes (short list*) |
| Janssen Research & Development USA | Replication-deficient adenovirus | PER.C6 | No | $456 million | Yes (short list*) |
| University of Pittsburgh | Protein subunit | HEK-293 | No | No | No |
| ImmunityBio/NantKwest | Replication-deficient adenovirus | HEK-293 or derivative E.C7 | No | No | Yes (long list) |
*The New York Times report
In four of the vaccines, the human fetal cells are used as miniature “factories” to generate vast quantities of adenoviruses, disabled so that they cannot replicate, that are used as vehicles to ferry genes from the novel coronavirus that causes COVID-19. When the adenoviruses are given as a vaccine, recipients’ cells begin to produce proteins from the coronavirus, hopefully triggering a protective immune response.
The fifth vaccine, which has shown promise in monkeys and is headed for human trials as soon as this summer, is what is known as a protein subunit vaccine. Researchers at the University of Pittsburgh use HEK-293 cells to manufacture the coronavirus’ spike protein—a vital part of its structure—which is used to trigger an immune response. The vaccine is delivered through a skin patch with 400 tiny needles.
The fetal cell lines are key to producing both types of vaccine. “HEK-293 [cells] are essential for making protein subunit vaccines,” says Andrea Gambotto, a vaccine scientist at the University of Pittsburgh School of Medicine and the vaccine’s lead developer. Their human origin is important, he says: “Cultured [nonhuman] animal cells can produce the same proteins, but they would be decorated with different sugar molecules, which—in the case of vaccines—runs the risk of failing to evoke a robust and specific immune response.” (Among the developers of the five vaccines, only Gambotto responded to a request for comment.)
David Prentice, vice president and research director at the Charlotte Lozier Institute, which opposes abortion, notes researchers making adenovirus vaccines have modified HEK-293 cells to be adept at packaging new genes—such as those that direct cells to assemble the coronavirus spike protein—into adenoviruses. But he adds that other technologies are available, including using cells captured from amniocentesis that are engineered to make replication-deficient adenoviruses.
“The use of cells from electively aborted fetuses for vaccine production makes these five COVID-19 vaccine programs unethical, because they exploit the innocent human beings who were aborted,” Prentice and a co-author—molecular biologist James Sherley, a Lozier Institute associate scholar and director of the adult stem cell company Asymmetrex—wrote in a position paper published last month.
But Arthur Caplan, a bioethicist at the New York University School of Medicine, counters: “There are better ways to win the abortion wars than telling people not to use a vaccine. These are long-over abortions. These cells are decades old, and even major religious leaders like the pope have acknowledged that for the greater good it’s not worth the symbolism to put the community at risk.”
The Vatican’s Pontifical Academy for Life declared in 2005 and reaffirmed in 2017 that in the absence of alternatives, Catholics could, in good conscience, receive vaccines made using historical human fetal cell lines.
A vaccine made by the Chinese company CanSino Biologics was the first COVID-19 vaccine to enter phase II human trials. It was developed using adapted HEK-293 cells that the company licensed from Canada’s NRC, where the cells were developed. (NRC-developed HEK-293 cells have already been used to develop an approved Ebola vaccine.) Last month, NRC announced a collaboration with CanSino Biologics under which it is preparing to run late-stage clinical trials of the vaccine in Canada, and scale up facilities to produce the vaccine in quantity.
The two U.S.-backed vaccines that have drawn criticism from antiabortion groups are on a short list of candidates targeted to get financial and logistical support from the U.S. government under the White House’s Operation Warp Speed, which aims to accelerate the development and approval of at least one COVID-19 vaccine by January 2021, according to a 3 June report in The New York Times.
One of the Warp Speed candidates, made by Janssen Research & Development, uses PER.C6 cells. The second, from University of Oxford researchers and AstraZeneca, uses HEK-293 cells. Both have received U.S. government commitments of, respectively, $456 million and $1.2 billion, if they meet milestones, through the Biomedical Advanced Research Development Authority (BARDA).
Another vaccine that relies on HEK-293, being developed by two companies owned by the billionaire scientist and businessman Patrick Soon-Shiong, made an earlier, Warp Speed long list of 14 promising candidates, according to a press release from one of companies, NantKwest.
Prentice says: “As they are choosing—BARDA and the Warp Speed people— what vaccines to move ahead, they should at least recognize that there is some portion of the population who would like an alternative vaccine they can take in good conscience.”
Caplan disagrees. “If you are going to say the government shouldn’t fund things that a minority of people object to, you will have a very long list of things that won’t get funded by the government, from research on weapons of war to contraceptive research.”
The Trump administration has restricted the use of human fetal tissue from elective abortions in biomedical research. One year ago, it adopted a policy that forbids researchers at the National Institutes of Health (NIH) from using fetal tissue from elective abortions in their studies. And it imposed an extra layer of review on non-NIH scientists seeking agency funding to do research using such tissue. But the policy did not stop either group from using decades-old fetal cell lines like HEK-293 and PER.C6.
*Clarification, 8 June, 12:10 p.m.: This story has been updated to clarify that the Vatican approves of Catholics receiving vaccines manufactured using human fetal cells only in the absence of alternatives.